Results: Poor responders: How to define, diagnose and treat?
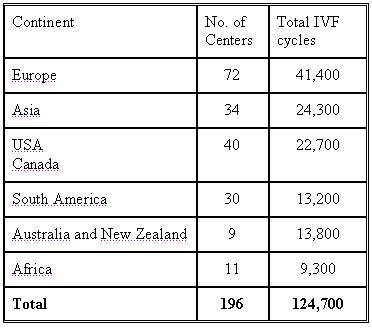
Results of 124,700 IVF treatment cycles (196 centres from 45 countries )
The statistics was done based on the number of cycles each unit performed and not on the number of units.
As can be seen below there is a much variation in the way treatment is being practiced reflecting lack of solid and evidenced-based data in this field.
The survey was compiled by:

Prof. Milton Leong, Director of IVF Center, Hong Kong Sanatorium and Hospital, Adjunct Professor of O+G, McGill University, Montreal, Canada

Prof. Pasquale Patrizio
Director Yale Fertility Center and REI medical practice from New Haven , CT , USA
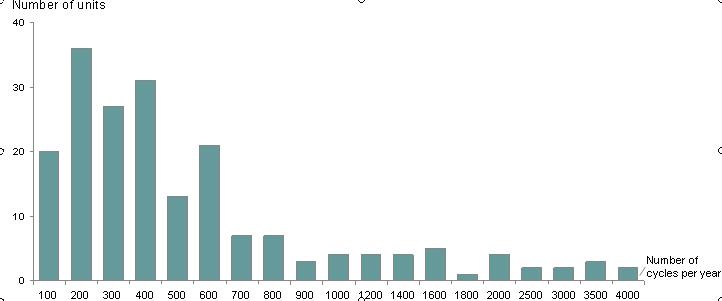
This graph describes the number of cycles performed by each unit participating in the survey. The majority of the units performed up to 400 cycles per year. There were only two units in this survey performing more than 4000 cycles per year.
Section 1: Definition of "Poor Responder" patients:
Should it based on ovarian response only?
Yes, Ovarian response only 68% (84,600cycles)
No 32% (40,100 cycles)
Should definition include endometrial response?
No – 86% (107,800 cycles)
Yes – 14% (16,900 cycles)
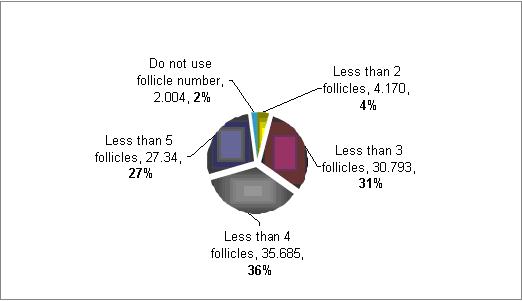
How you define poor responders based on number of follicles?
The majority (71%) defined poor responders patients as those with less then 4 follicles at the time of aspiration . However, there is a much variation in between 3 to 5 follicles.
How you define poor responders based on number of follicles?
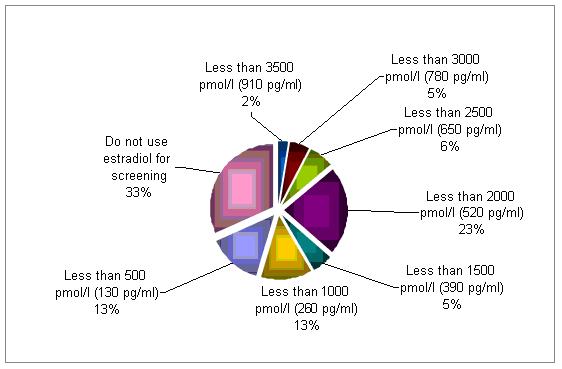
Serum levels of estradiol are being used to corroborate a diagnosis of poor responder by 67% of the cycles evaluated . Although there is no clear cut off, a serum concentration below 2500 pmol/l (650 pg/ml) raise a concern.
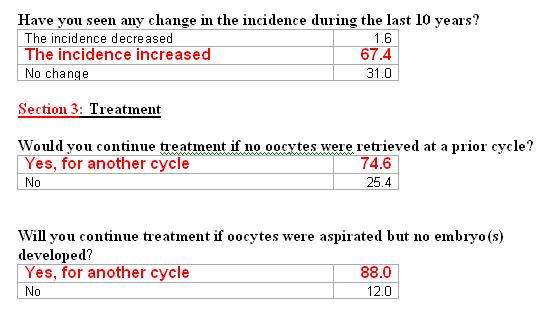
Is History important? Previous performance before and during IVF?
Yes – 95.8%
No- 4.2%
Section 2: Screening methods for diagnosis
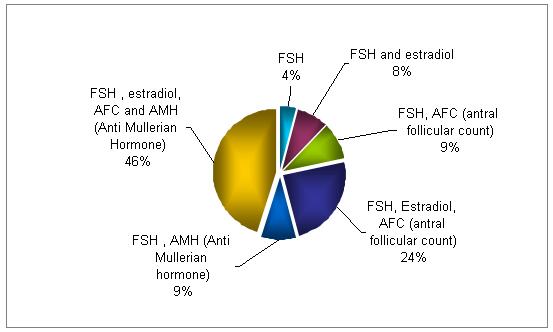
What screening methods do you use to identify poor responders?
The combination of FSH, E2, and AFC is used in 70% of the testing for identifying poor responders. The quadruple testing (FSH, E2, AFC and AMH ) is used in 46% of the cycles.
Do you measure FSH/LH ratio?
No – 62.7%
Yes – 37.3%
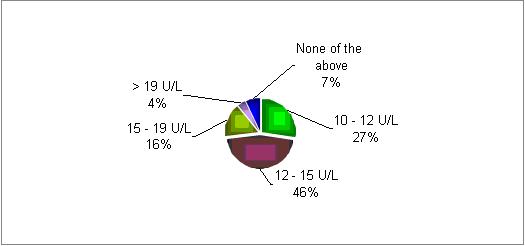
At what level of day 2 FSH would you identify a poor responder?
In most of the responding centers there is a decrease in patients' response if the initial FSH level on day two is above 10 U/L.
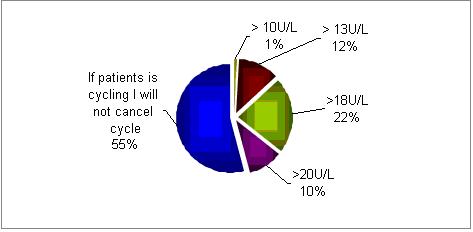
In normally cycling patients, would you cancel IVF treatment if day 2 FSH is?
In most centers (55%) if the patients is still cycling the FSH serum concentrations are not taken into account. However, if FSH values are being considered, then 18U/L is the cut-off point in the majority of centers.
Do you do any dynamic testing?
Clomiphene Citrate challenge test (CCCT) – 19.6%
GnRH agonist plus Clomiphene Citrate challenge test (CCCT) – 2.2%
No – 88.2%
Do you use the ultrasound for diagnosis?
3-D Ultrasound and Doppler study 0.5
Antral follicle count 88.0%
Both 5.8%
None of the above 5.7%
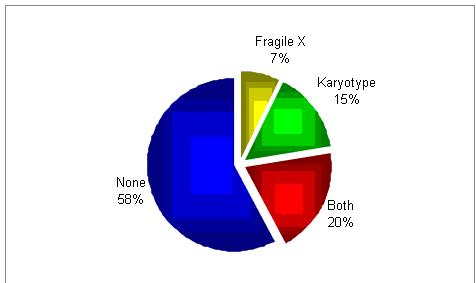
Would you do genetic testing?
It seems as if there is no a common practice.
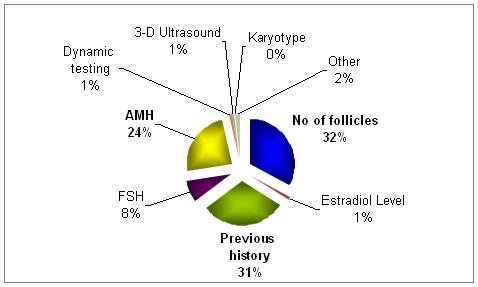
What, in your opinion and experience, is the most important predictor of the above?
The most important predictor factors are the number of follicles developed along with the history.
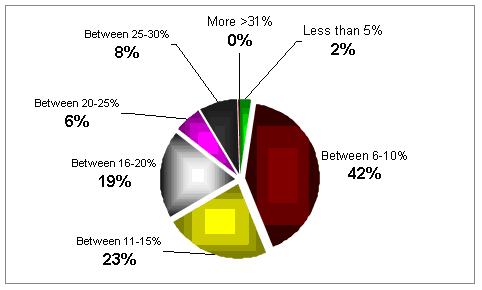
Can you estimate the scale of poor responders in your clinic?
In most of the clinics the percentage is quite high, between 6 to 15%.
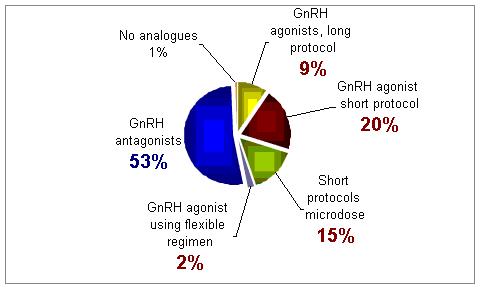
Of the following GnRH analogues protocols, which one do you use more often for poor responders?
For this group of patients treatment with GnRH antagonist was selected as the best treatment approach
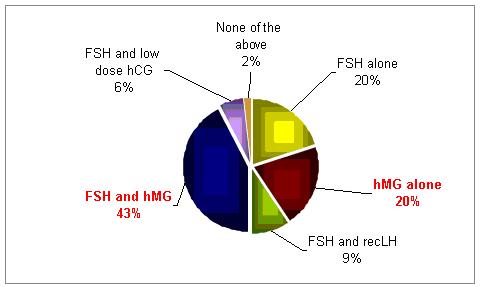
What combination of gonadotropins do you use?
In poor responder patients the use of hMG with FSH or hMG alone were found to be the most common forms of treatment.
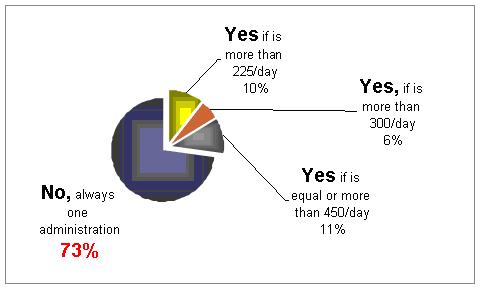
Would you divide the daily dose into two administrations?
It is not clear what should be the methods. 27% would divide the doses into 2 injections.
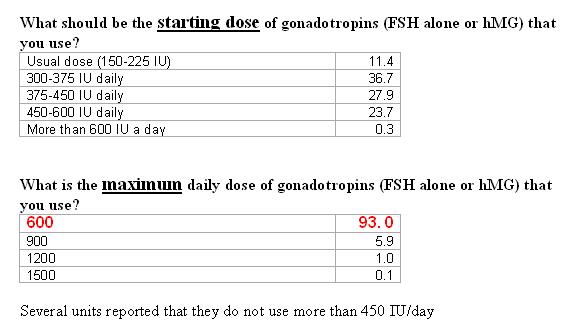
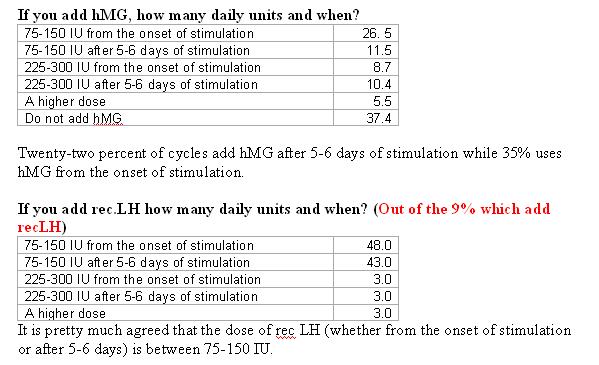
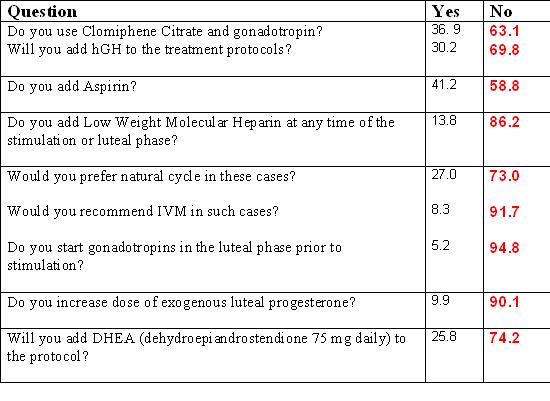
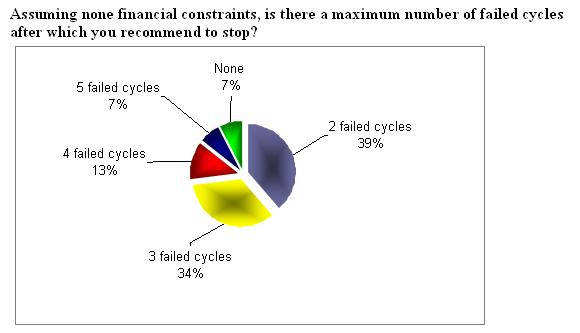
How long will you continue with the maximum dose, if there is no response, before you will stop treatment (cycle cancellation)?
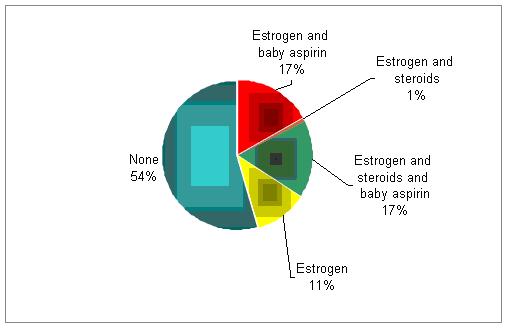
In addition to progesterone, do you support the luteal phase with estrogen, aspirin, or steroids?
Comments received to the survey:
- Genetic testing only if patient is younger than 40 years.The vast majority of poor responders, who have adequate BAF, tend to have an absolute or relative deficiency of FSH. Meaning there is a hormone transmission defect in capillary occlusion with clotting imbalance or vasospasm or missing cofactor IGF-1 (GH)We use rGH. - 450IUWe use FSH as one bit of info.If 2 cycles failed we propose oocyte donation for our patientsWe use herbal medicines before the stimulationLetrozole can be used with hMG (instead of Clomiphine)We prefer natural cycle in most cases, and add cycle by cycle oocytes using Kuwayama´s Vitrifications.Predicting poor response is different from diagnosis of poor response. 300IU is our maximum dose.Several units reported that the highest dose of gonadotropins would be 450 IU/dayNatural cycle with add back LH and antagonist works for someAssisted Hatching only added if embryo itself need it. There is no medical decision but counselling.The best way to define poor responders is to see how they respond to medicationsThere is still no clear cut results in poor responders
The below comment was received from Mark Perloe
Georgia Reproductive Specialists, This email address is being protected from spambots. You need JavaScript enabled to view it.
IVF patients who will receive late luteal phase Lupron and have previously demonstrated poor response or have been on prolonged Lupron therapy are offer the GRS-PREP protocol. Birth control pills will be utilized for 7-21 days prior to initiating Lupron. Administration of Lupron 10 units SQ will be initiated after a minimum of 7 days on birth control pills. Birth control pills will continue for 5-7 days after Lupron initiation. A baseline ultrasound and serum estradiol will be obtained 4-7 days off birth control pills. If the endometrium is thin and the estradiol ≤65pg/ml, the patient will receive estradiol valerate 2mg IM and drop Lupron dose to 5 units. Five days later, gonadotropin administration will begin. [A visit may be scheduled on the day of gonadotropin initiating for protocol review if either the physician or IVF nurse believe it will be to the patients benefit.] An estradiol 0.1mg patch will be placed on the day gonadotropins are initiated. Patches will be changed every 3 days and will continue until multiple follicles greater than 10-12mm are noted.
We noticed that when oral contraception is utilized to time a ganerelix cycle, those who start a stimulation on the second or third day off the pills tend to have longer stimulation cycles using considerable more medication and have fewer eggs. However, results seem better if you wait to the fourth of fifth day after discontinuing the pill. We hypothesized that this might be related to down-regulation of gonadotropin receptors by Lupron and the pill and as outcomes seem improved by delaying the outcome until the fourth or fifth day of estrogen exposure, a brief exposure to estrogen by promote restoration of gonadotropin receptors. We have read with interest Geoffrey Sher's AACEP protocol in poor responders and believe that the perceived benefit may be related to up-regulation of receptors by estrogen and not the use of both analogue and antagonist. While data from controlled studies for either the AACEP protocol or the GRS-PREP protocol are not available, anecdotally, we have seen better results when this approach is utilized in poor responders. Further randomized clinical trials must be carried out to determine whether this perceived benefit is simply related to regression toward the mean.
On behalf of the IVF-Worldwide team Prof. Fauser, Dr. Tur-Kaspa and the Advisory Board of the website, we would like to take this opportunity to thank all the centers that participated in the survey by adding to the global knowledge, as this could be of use around the world.